利用宏观和微观电化学方法了解不锈钢热喷涂涂层的腐蚀行为
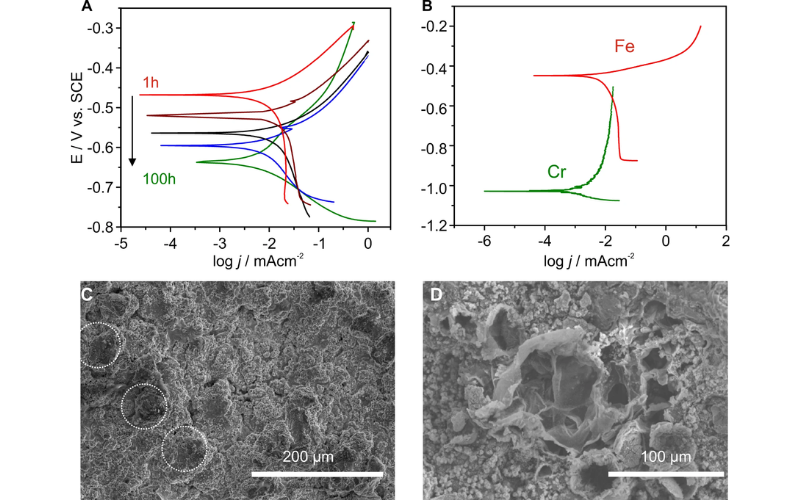
高速氧气燃料热喷涂不锈钢涂层因其出色的耐侵蚀性而备受青睐。然而,与原始块状材料相比,制造过程会导致耐腐蚀性能下降。在此,我们使用不同的沉积参数在不锈钢基底上制作不锈钢涂层,以研究由此产生的复合钢的腐蚀性能,并阐明其在宏观和微观尺度上的腐蚀行为。在腐蚀环境中进行的宏观电位极化测量显示了铁铬合金涂层的降解速度。在短时间浸泡后,涂层显示出类似铁的活性腐蚀行为,阳极分支上没有钝化区。随着时间的推移,涂层的腐蚀行为开始发生变化,显示出与纯铬相似的结果。原位电子显微镜和元素组成显示,涂层表面留下了富含氧化铬的层。在涂层和粉末材料上分别采用了扫描电化学显微镜和扫描微量移液管接触法等微电化学技术,结果表明,热喷涂涂层所具有的保护钝性不足主要是由雾化的粉末不锈钢材料所继承的。
原文发表于《npj Materials Degradation》(第3卷,文章编号:25(2019))
作者:Samantha Michelle Gateman, Ilias Halimi, Alexandre Romão Costa Nascimento, Robert Lacasse, Robert Schulz, Christian Moreau, Richard Chromik, Janine Mauzeroll